Cliantha Research is a global CRO dedicated to applying good science and research integrity into the development of much needed medications. Here in our blog, we will share our insights from our considerable experience in clinical research at all phases of clinical development.
Topics Covered & Coming Soon…
- Innovative Approaches for Innovative Drugs
- Phase III Clinical Trial on Allergic Conjunctivitis performed in an Environmental Exposure Chamber
- Nasal Spray Fentanyl for Break Through Cancer Pain: A Question of Bioavailability in Patients with Rhinitis
- Non-Allergic Rhinitis: Provocateurs of Disease other than Allergens
- Dry Eye Disease & Contact Lens-Induced Dry Eye: The controlled study of changes in the lens and ocular surface in a low humidity environment
- Accelerated Phase I
- Fast-To-File Approaches
- A First-To-File Approach: A Case Study
- The Cliantha Approach to Clinical Equivalence Trials = North America + India
- When Bigger is Not Better – Why a mid-sized CRO is better for small to medium sized Biotechs
Allergy
Innovative Approaches to Clinical Development of Innovative Drugs: A Phase III Allergy Study Conducted in an Environmental Exposure Chamber (EEC)
After having successfully surmounted the considerable research hurdles of demonstrating good product safety in Phase I, efficacy and dose-ranging in Phase II, establishing a regulatory-acceptable Phase III trial design (which reduces the risk of not meeting your primary endpoint) can be daunting. In particular, this is the case in clinical trials for allergy.
Traditional approaches include studying patients’ subjective responses to drug or placebo treatment in their everyday lives, relying on environmental allergen exposures. Relying on allergen exposure naturally is particularly risky of late as a result of global climate change.
For more information on the impact of climate change on respiratory studies the following presentations from AAAAI and EAACI may be helful:
The Risks of Field Trials in Allergy & Asthma
Climate change has resulted in unpredictable pollen release that fails to elicit allergy symptoms in allergy patients enrolled in a clinical trial. If patients do not develop symptoms, then a drug’s effectiveness on allergy symptoms cannot be reliably tested, with both placebo and active treatments showing a similar symptom level. Other sources of variability in this approach are patient lifestyles, which result in very different levels of allergen exposures. For example, a gardener who spends most working hours outdoors and is highly exposed to pollen, contrasts greatly with an office worker who spends very little time outdoors and is exposed to low allergen levels due to highly conditioned and filtered air in office buildings.
How to De-risk your Phase III Allergy Trials
As a scientist who has been involved in research from the Bench through to the Bedside for over 20 years, one manner in which to improve the ability of your clinical trial to be discriminative, and thereby to give you good quality outcomes, is to design the study to reduce intra- and inter-subject variability. One way to do this in allergy trials is to study your patients with a controlled allergen challenge. At Cliantha, we have developed Environmental Exposure Chambers or EECs which are specialized facilities in which the allergen of interest is aerosolized at levels that mimic a typical peak pollen day for seasonal allergens or a typical exposure seen indoors for other allergens like house dust mite or cat allergens. Our EECs are built to facilitate the aerosolization of allergens in a controlled fashion, whilst still having some variability that mimics nature while doing so safely with appropriate patient monitoring. The EEC truly brings together the best of both the scientific and clinical worlds with a controlled exposure to allergen that mimics real-world allergen level exposures. (See below one of our EECs located in Toronto, Canada).
- Dr. Salapatek has received grants for her research from the Canadian Institutes of Health Research, NIH-NIAID, and Banting & Best Diabetes Research Unit to name a few, and has overseen over one hundred clinical trials Phases I through Phase IV for drug submissions to regulatory agencies including but not limited to FDA, Health Canada, MHRA and European Medicines Agency (EMA). Dr. Salapatek now serves as the Chief Scientific Officer and Executive Vice President of Cliantha Research and a Faculty Lecturer at McMaster University.
 It is important to note that when subjects are carefully screened for their allergies and they are exposed to a controlled naturalistic, airborne allergen in the EEC, subjects who are not allergic do not develop symptoms psychosomatically. Cliantha has conducted testing over the years to demonstrate that those subjects who are not allergic do not develop symptoms, while those who truly are allergic to the allergen being tested can have a spectrum of response. We often observe, for example, that subjects may have a large skin prick test response to a given allergen, but do not report “clinically relevant” symptoms in their everyday lives and when these subjects are studied in the EEC, they do not develop many symptoms. For a successful clinical trial, subjects should demonstrate a moderate to high level of symptoms.As a scientist who has been involved in research from the Bench through to the Bedside for over 20 years, one manner in which to improve the ability of your clinical trial to be discriminative, and thereby to give you good quality outcomes, is to design the study to reduce intra- and inter-subject variability. One way to do this in allergy trials is to study your patients with a controlled allergen challenge. At Cliantha, we have developed Environmental Exposure Chambers or EECs which are specialized facilities in which the allergen of interest is aerosolized at levels that mimic a typical peak pollen day for seasonal allergens or a typical exposure seen indoors for other allergens like house dust mite or cat allergens. Our EECs are built to facilitate the aerosolization of allergens in a controlled fashion, whilst still having some variability that mimics nature while doing so safely with appropriate patient monitoring. The EEC truly brings together the best of both the scientific and clinical worlds with a controlled exposure to allergen that mimics real-world allergen level exposures. (See below one of our EECs located in Toronto, Canada).
It is important to note that when subjects are carefully screened for their allergies and they are exposed to a controlled naturalistic, airborne allergen in the EEC, subjects who are not allergic do not develop symptoms psychosomatically. Cliantha has conducted testing over the years to demonstrate that those subjects who are not allergic do not develop symptoms, while those who truly are allergic to the allergen being tested can have a spectrum of response. We often observe, for example, that subjects may have a large skin prick test response to a given allergen, but do not report “clinically relevant” symptoms in their everyday lives and when these subjects are studied in the EEC, they do not develop many symptoms. For a successful clinical trial, subjects should demonstrate a moderate to high level of symptoms.As a scientist who has been involved in research from the Bench through to the Bedside for over 20 years, one manner in which to improve the ability of your clinical trial to be discriminative, and thereby to give you good quality outcomes, is to design the study to reduce intra- and inter-subject variability. One way to do this in allergy trials is to study your patients with a controlled allergen challenge. At Cliantha, we have developed Environmental Exposure Chambers or EECs which are specialized facilities in which the allergen of interest is aerosolized at levels that mimic a typical peak pollen day for seasonal allergens or a typical exposure seen indoors for other allergens like house dust mite or cat allergens. Our EECs are built to facilitate the aerosolization of allergens in a controlled fashion, whilst still having some variability that mimics nature while doing so safely with appropriate patient monitoring. The EEC truly brings together the best of both the scientific and clinical worlds with a controlled exposure to allergen that mimics real-world allergen level exposures. (See below one of our EECs located in Toronto, Canada).
Skin Prick Testing (SPT) @ Cliantha Research is only part of the allergy testing done to test allergen sensitivity.
 At Cliantha, we have developed chambers to study participants safely and naturally for a variety of allergens: both seasonal pollen allergens such as trees (birch & oak), grass (mix of grasses, including Timothy grass, which is the major grass species), and ragweed, as well as perennial allergens, such as house dust mite allergen and cat allergen.
At Cliantha, we have developed chambers to study participants safely and naturally for a variety of allergens: both seasonal pollen allergens such as trees (birch & oak), grass (mix of grasses, including Timothy grass, which is the major grass species), and ragweed, as well as perennial allergens, such as house dust mite allergen and cat allergen.
How does it work?
In chamber studies, you can screen participants for their level of symptoms. This improves clinical outcomes as we only study patients with adequate level of symptoms.
Both prophylactic treatment and on-symptom treatment can be studied in the chamber. Prophylactic treatments that are long-acting are studied such that you can follow-up at different times after treatment and can assess the duration of the response to the treatment. This is particularly helpful for drugs such as corticosteroids or immunotherapies that are expected to alter the underlying causes of disease and thereby can have long-standing effects.
The chamber is also well-placed for the study of fast-acting drugs. At Cliantha, we recently performed a Phase IV study for Dymista© showing the fastest onset ever demonstrated for this drug class, an onset of 5 minutes! See our recent publication in JACI in Practice in 2018 (JACI In Practice 6(5):1726,2018). In addition, we have conducted 12-hour long chamber sessions or had another chamber session early the next day, so that we could establish the duration of action of a drug for label claims. In our 2010 paper, published in the American Journal of Rhinology & Allergy, we showed an early onset of action of mometasone furoate after a single treatment and duration over 24 hours after 7 days daily treatment (see Salapatek A, Patel P, Gopalan G, Varghese S. Mometasone furoate nasal spray provides early, continuing relief of nasal congestion and improves nasal patency in allergic patients. Am J Rhinol Allergy 2010; 24:433-8.)
Chambers can be used in the same way for other diseases such as Dry Eye disease or Non-Allergic Rhinoconjunctivitis (NAR). Here the provocateurs are different, but the approach is the same. For example, dry air directed at the ocular surface can be used to exacerbate the signs and symptoms of dry eye disease. Patients can be studied under these drying conditions, before and after treatment, to see how the ocular treatment may help to reduce the symptoms developed in the EEC. For NAR, common provocateurs that act as environmental irritants can be studied such as ozone, which is a component of air pollution that can lead to symptoms in subjects’ everyday lives. This is a significant unmet medical need. We will talk more directedly about this in upcoming blogs… Stay tuned.
The concern to-date has been that EEC approach, whilst good for dose-finding, may not correlate well with everyday exposures. Our recent work shows that there is a high degree of correlation in treatment effect in field and EEC. This is particularly enhanced when you compare those highly exposed in the field to their response in the EEC. This intuitively makes sense as if you are not exposed in the field i.e. office worker with little time outdoors, your symptom level would be lower and therefore cannot show drug effect.
 At the upcoming 2019 World Allergy Congress in Lyon, France, Cliantha will be presenting a study in which participants were studied both in the EEC with grass pollen and in their everyday lives during the grass pollen season. Important to note patients were reporting their symptoms both in the EEC and in their everyday lives using Cliantha’s electronic Data Acquisition Tablet (ePDAT©), which is a 21CFR Part 11 compliant and validated electronic Patient Reported Outcomes (ePRO) tool. In the graphs below, you see that participants who reported more than 3 hours outside and significant symptom development outside had the highest significant correlation to their symptoms developed in the EEC, in contrast to those participants who reported less than 3 hours outdoors. It can be seen that when we compare “apples to apples” there is (as we would expect) a significant and high correlation between symptoms in the EEC and the field. See figure below which shows this nicely.
At the upcoming 2019 World Allergy Congress in Lyon, France, Cliantha will be presenting a study in which participants were studied both in the EEC with grass pollen and in their everyday lives during the grass pollen season. Important to note patients were reporting their symptoms both in the EEC and in their everyday lives using Cliantha’s electronic Data Acquisition Tablet (ePDAT©), which is a 21CFR Part 11 compliant and validated electronic Patient Reported Outcomes (ePRO) tool. In the graphs below, you see that participants who reported more than 3 hours outside and significant symptom development outside had the highest significant correlation to their symptoms developed in the EEC, in contrast to those participants who reported less than 3 hours outdoors. It can be seen that when we compare “apples to apples” there is (as we would expect) a significant and high correlation between symptoms in the EEC and the field. See figure below which shows this nicely.
The rhinoconjunctivitis symptoms developed in an EEC correlate highly and significantly with symptoms developed in the field when the participant reports that they have spent some time outdoors.
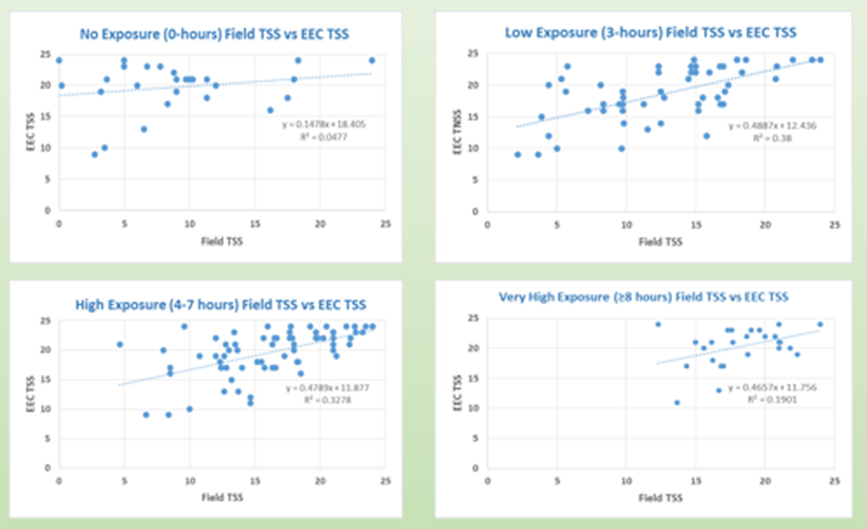 Figure (above) will be presented at the World Allergy Congress 2019 and shows the following:
Figure (above) will be presented at the World Allergy Congress 2019 and shows the following:
- Average Total Symptom Score for the same subjects who reported no exposure in the field showed no correlation (r=0.218, p=0.274).
- Subjects who reported daily outdoor exposure of 0-3 hours had the strongest significant positive correlation between Field and EEC (r=0.616, p<0.0001).
- Subjects who were exposed for 4-7 hours outside also had a highly significant strong positive correlation (r= 0.5726, p<0.0001).
- Subjects exposed ≥8 hours daily reported the highest TSS however while statistically significant, less positive correlation was observed (r= 0.436, p<0.023).
are you going to the world allergy congress 2019 in lyon, france? we would love to meet you and talk about your drug development.
To schedule a meeting please contact Cynthia day O’Brien senior director of Business development at cliantha research at
cobrien@cliantha.com
A Case Study:
A Phase III Allergic Conjunctivitis Trial Performed in the EEC
On October 31, 2019 8:00 AM EDT Aldeyra Therapeutics presented an “Expanded Data Release Presentation”, in which the clear signal shown in their Phase II EEC study to demonstrate how their lead product candidate, reproxalap, markedly reduced the signs and symptoms of allergic conjunctivitis. Listen to this presentation for further details at https://ir.aldeyra.com/events. This study was performed in Cliantha Research’s allergen chamber. The figure below shows some details:
 Reproxalap is Aldeyra’s lead candidate and is an innovative drug that is first-in-class acting on pro-inflammatory molecules called RASPs, which are elevated in a range of inflammatory diseases including dry eye disease and allergic conjunctivitis.
Reproxalap is Aldeyra’s lead candidate and is an innovative drug that is first-in-class acting on pro-inflammatory molecules called RASPs, which are elevated in a range of inflammatory diseases including dry eye disease and allergic conjunctivitis.
On November 7th, Aldeyra’s CEO, Dr. Todd Brady announced during their quarterly report that Aldeyra had reached an agreement with FDA on their innovative Phase III study called INVIGORATE to study reproxalap, for the treatment of Allergic Conjunctivitis. Listen to this webcast:
click hear Uniquely, INVIGORATE will be performed in Cliantha’s allergen chamber or EEC. This validates the approach of the EEC for drug testing in Phase III. He described how the Phase II study had informed the Phase III study and how they “will use the allergen chamber, an innovative and rigorous method to test in allergic conjunctivitis that is optimal for testing drugs in allergic conjunctivitis.”
Dr. Brady described from research in Current Opinion Allergy and Clinical Immunology that 40% of population in the US alone suffer from anterior segment ocular disease and that this accounts for $11B in prescription drugs. Specifically, he reported that 30M people suffer from allergic conjunctivitis in the US that do not respond to or are dissatisfied with mainstay treatment of antihistamines and therefore need a new approach.
The safety of ocular reproxalap is well established to date, as over 1,000 patients have been dosed across 12 studies with no observed safety concerns. Many medical practitioners are keen to have a new approach for allergic conjunctivitis. These results have been presented at the AAO and Current Opinions in Ophthalmology this year.
What this Case Study shows:
- EEC can be used to screen patients rigorously for the signs and symptoms of allergy.
- The EEC combines the real-world exposure of a field trial with the controlled exposure in an allergen chamber.
- EEC can be used to look at onset and durability of action of putative drugs.
- Regulatory acceptance for pivotal EEC trials.
These observations, coupled with the practical aspects of EECs, act to reduce the risk of a failed allergy trial and provide cost and time efficiencies, making this a compelling research approach and option for your drug development program.
DR. ANNE MARIE SALAPATEK
Executive VP & Chief Science Officer
asalapatek@cliantha.com